Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
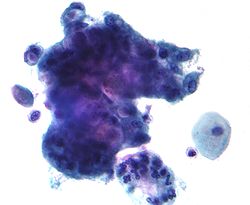
Mucin
 | |
| Identifiers | |
|---|---|
| Symbol | Mucin |
| Membranome | 111 |
Mucins (/ˈmjuːsɪn/) are a family of high molecular weight, heavily glycosylated proteins (glycoconjugates) produced by epithelial tissues in most animals.[1] Mucins' key characteristic is their ability to form gels; therefore they are a key component in most gel-like secretions, serving functions from lubrication to cell signalling to forming chemical barriers.[1] They often take an inhibitory role.[1] Some mucins are associated with controlling mineralization, including nacre formation in mollusks,[2] calcification in echinoderms[3] and bone formation in vertebrates.[4] They bind to pathogens as part of the immune system. Overexpression of the mucin proteins, especially MUC1, is associated with many types of cancer.[5][6]
Although some mucins are membrane-bound due to the presence of a hydrophobic membrane-spanning domain that favors retention in the plasma membrane, most mucins are secreted as principal components of mucus by mucous membranes or are secreted to become a component of saliva.
- ^ a b c Marin F, Luquet G, Marie B, Medakovic D (2007). Molluscan shell proteins: primary structure, origin, and evolution. Current Topics in Developmental Biology. Vol. 80. Academic Press. pp. 209–76. doi:10.1016/S0070-2153(07)80006-8. ISBN 9780123739148. PMID 17950376.
- ^ Marin F, Corstjens P, de Gaulejac B, de Vrind-De Jong E, Westbroek P (July 2000). "Mucins and molluscan calcification. Molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, pteriomorphia)". The Journal of Biological Chemistry. 275 (27): 20667–20675. doi:10.1074/jbc.M003006200. hdl:1887/50061. PMID 10770949.
- ^ Boskey AL (2003). "Biomineralization: an overview". Connective Tissue Research. 44 Suppl 1 (1): 5–9. doi:10.1080/713713622. PMID 12952166.
- ^ Midura RJ, Hascall VC (October 1996). "Bone sialoprotein--a mucin in disguise?". Glycobiology. 6 (7): 677–681. doi:10.1093/glycob/6.7.677. PMID 8953277.
- ^ Niv Y (April 2008). "MUC1 and colorectal cancer pathophysiology considerations". World Journal of Gastroenterology. 14 (14): 2139–2141. doi:10.3748/wjg.14.2139. PMC 2703837. PMID 18407586.
- ^ Brockhausen I, Melamed J (August 2021). "Mucins as anti-cancer targets: perspectives of the glycobiologist". Glycoconjugate Journal. 38 (4): 459–474. doi:10.1007/s10719-021-09986-8. PMID 33704667. S2CID 232191632.
Previous Page Next Page


