Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Heme

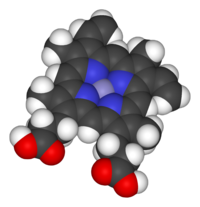
Heme (American English), or haem (Commonwealth English, both pronounced /hi:m/ HEEM), is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains.[1] Heme is biosynthesized in both the bone marrow and the liver.[2]
Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including metabolism of some drugs), gas sensing (guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8).[3][4]
Heme is a coordination complex "consisting of an iron ion coordinated to a tetrapyrrole acting as a tetradentate ligand, and to one or two axial ligands".[5] The definition is loose, and many depictions omit the axial ligands.[6] Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used[7] and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase.[8][9]
The word haem is derived from Greek αἷμα haima 'blood'.
- ^ Hodgson E, Roe RM, Mailman RB, Chambers JE, eds. (2015). "H". Dictionary of Toxicology (3rd ed.). Academic Press. pp. 173–184. doi:10.1016/B978-0-12-420169-9.00008-4. ISBN 978-0-12-420169-9. Retrieved 2024-02-21.
- ^ Bloomer JR (1998). "Liver metabolism of porphyrins and haem". Journal of Gastroenterology and Hepatology. 13 (3): 324–329. doi:10.1111/j.1440-1746.1998.01548.x. PMID 9570250. S2CID 25224821.
- ^ Dutt S, Hamza I, Bartnikas TB (2022-08-22). "Molecular Mechanisms of Iron and Heme Metabolism". Annual Review of Nutrition. 42 (1): 311–335. doi:10.1146/annurev-nutr-062320-112625. ISSN 0199-9885. PMC 9398995. PMID 35508203.
- ^ Ogun AS, Joy NV, Valentine M (2024), "Biochemistry, Heme Synthesis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30726014, retrieved 2024-02-22
- ^ Chemistry IU (2009). "Hemes (heme derivatives)". IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.H02773. ISBN 978-0-9678550-9-7. Archived from the original on 22 August 2017. Retrieved 28 April 2018.
- ^ A standard biochemistry text defines heme as the "iron-porphyrin prosthetic group of heme proteins"(Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.)
- ^ Poulos TL (2014-04-09). "Heme Enzyme Structure and Function". Chemical Reviews. 114 (7): 3919–3962. doi:10.1021/cr400415k. ISSN 0009-2665. PMC 3981943. PMID 24400737.
- ^ Paoli M (2002). "Structure-function relationships in heme-proteins" (PDF). DNA Cell Biol. 21 (4): 271–280. doi:10.1089/104454902753759690. hdl:20.500.11820/67200894-eb9f-47a2-9542-02877d41fdd7. PMID 12042067. S2CID 12806393. Archived (PDF) from the original on 2018-07-24.
- ^ Alderton W (2001). "Nitric oxide synthases: structure, function and inhibition". Biochem. J. 357 (3): 593–615. doi:10.1042/bj3570593. PMC 1221991. PMID 11463332.
Previous Page Next Page


